Recommended evaluation and testing before LYNKUET® initiation1
- Exclude pregnancy in females of reproductive potential
- Perform baseline hepatic laboratory tests to evaluate for hepatic function and injury [including serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum alkaline phosphatase (ALP), and serum bilirubin (total and direct)] before initiating treatment with LYNKUET. Do not start LYNKUET if ALT or AST is ≥2 times upper limit of normal (ULN) or if the total bilirubin is ≥2 times ULN
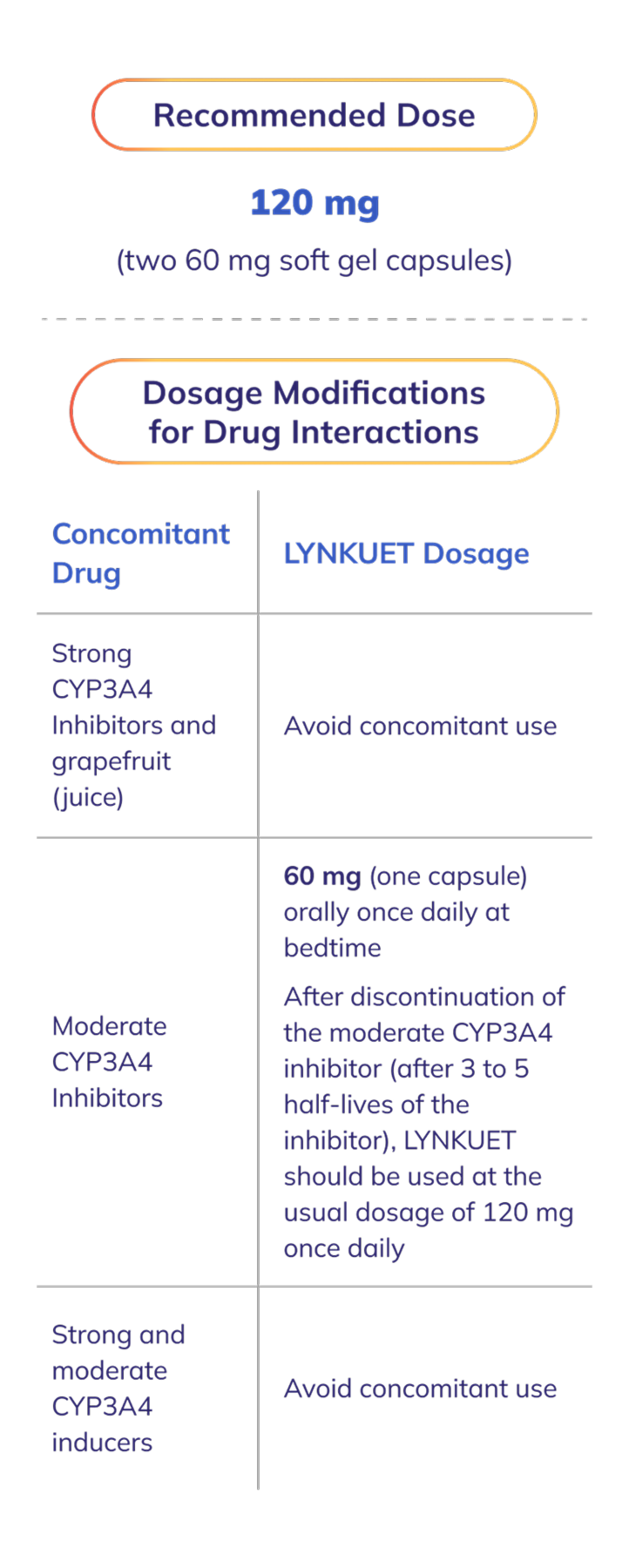
Recommended dosage
The recommended dosage of LYNKUET is 120 mg (two 60 mg capsules) orally once daily at bedtime at about the same time each day1
If a dose is missed at bedtime, the next dose should be taken as scheduled on the following day1
Two doses should not be taken on the same day to make up for a missed dose1
LYNKUET can be taken with or without food. Capsules should be taken with water and swallowed whole. Do not cut, crush, or chew capsules1

- Perform follow-up evaluations of hepatic transaminase concentration 3 months after initiation of therapy1
- Advise patients to discontinue LYNKUET immediately and seek medical attention, including hepatic laboratory tests, if they experience signs or symptoms that may suggest liver injury (new onset fatigue, decreased appetite, nausea, vomiting, pruritus, jaundice, pale feces, dark urine, or abdominal pain)1
- Discontinue LYNKUET if transaminase elevations exceed five times the ULN or if transaminase elevations exceed three times the ULN and total bilirubin exceeds two times the ULN1
- Advise patients who experience somnolence and other nervous system effects to refrain from driving or engaging in hazardous occupations or activities until the effects have resolved1
See the Dosing and Administration Summary


